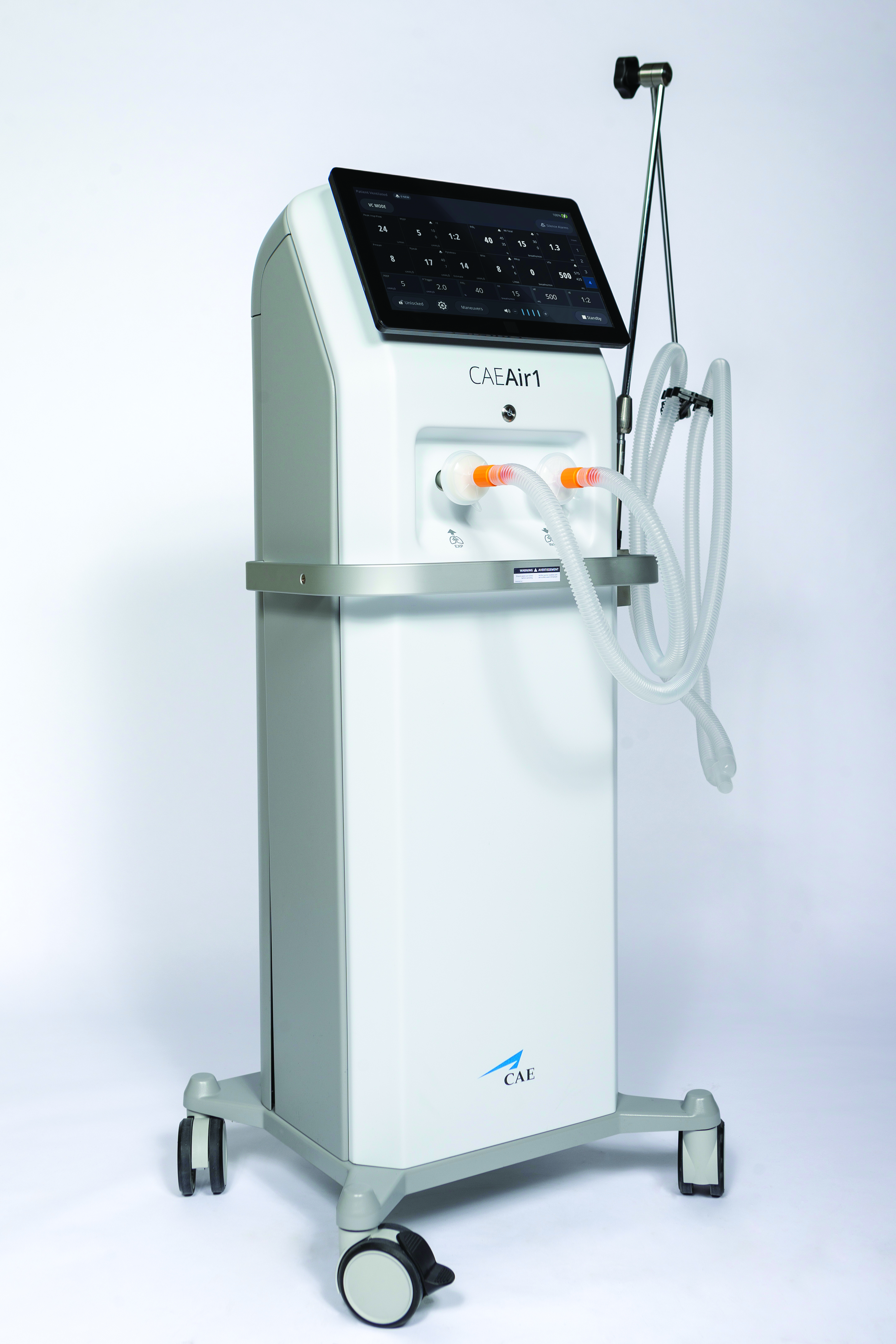
In April 2020, following the shutdown in response to the COVID-19 pandemic, CAE Healthcare responded to the Canadian government’s call for the production of 10,000 ventilators. CAE was known for producing simulation equipment for the medical and aviation industries, but they transformed their manufacturing model to move into full-scale ventilator mass production.
Unlike other ventilator projects using existing designs, CAE opted to engineer and develop a new ventilator, the CAE Air1. It began as the size of a shoe box and was meant to be a portable unit before evolving into a high-end, intensive care ventilator unit measuring approximately four feet tall. Thermoformer Plastique Art was up to the challenge of producing the parts required for the machine.
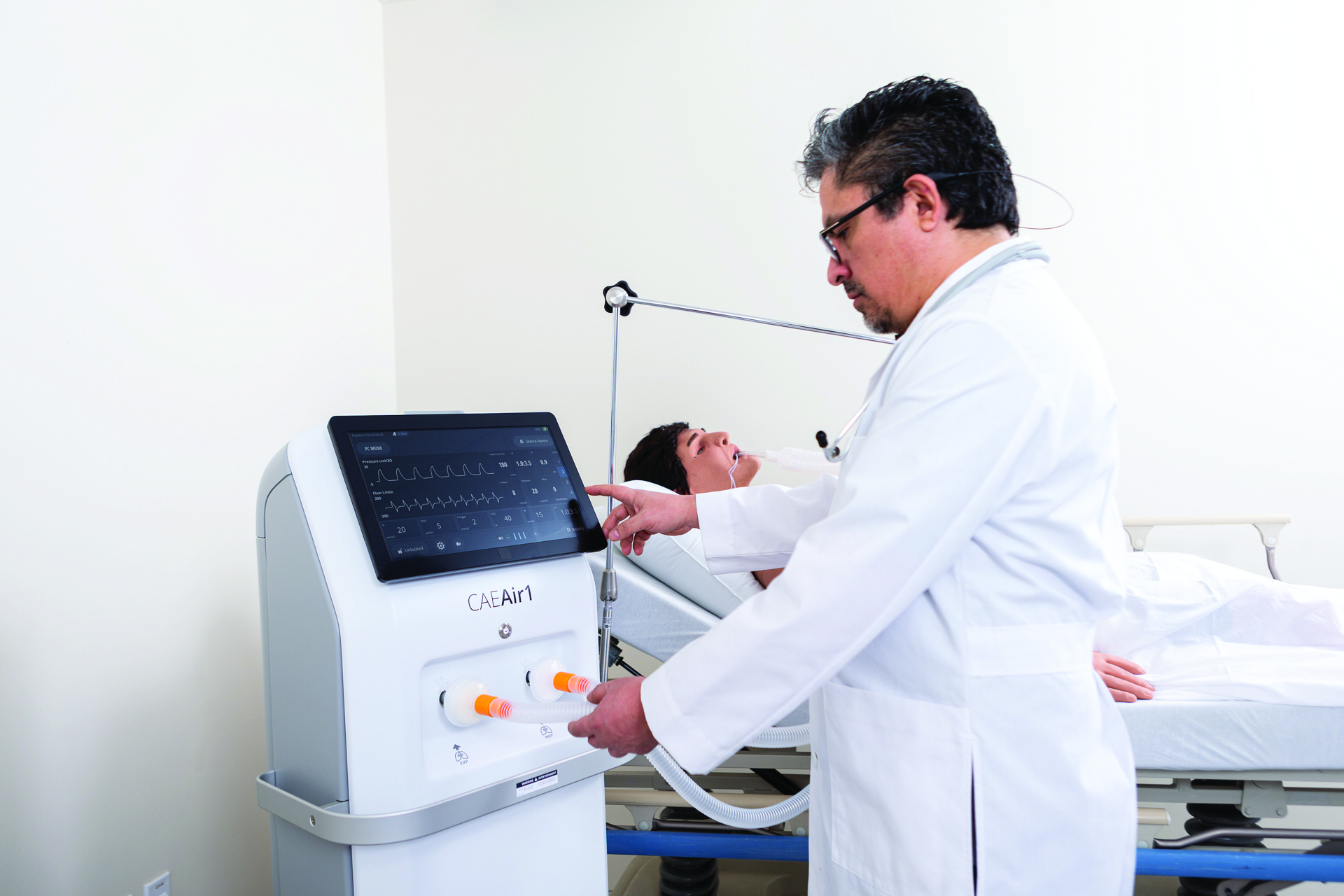
As experts in the field, Plastique Art provided guidance on recommended materials and thermoforming processes. The project required medical grade materials that were durable, chemical resistant and met the demand of certification from Health Canada. Plastique Art turned to KYDEX® Thermoplastics, knowing they were widely used in medical applications.
Using technical data and material testing information from SEKISUI KYDEX, Plastique Art was able to expedite the material choice with CAE. KYDEX Thermoplastics are durable, chemical resistant, inherently antimicrobial and available in a wide range of options for design. Ultimately, Plastique Art chose KYDEX T for the project.
Housing choices considered for the CAE Air1 ventilator included 3D printing, injection molding and sheet metal. However, when considering the urgency and volume of the project, Plastique Art knew thermoforming, specifically vacuum forming, was the best solution. 3D printed parts and sheet metal solutions are labor intensive to fabricate. They also require extensive finishing processes to seal, sand and paint to achieve the same level of aesthetics of thermoplastic material with in-mold color features.
Vacuum forming allows for shorter lead times to have material on hand for processing, shortening the production time for parts. Tools can be turned around in under two weeks, while pressure forming tools may take four to six. Once the sheet is formed, it only requires trimming and minimal assembly time for installation.
Final CAD files were received on April 20 and by April 30, Plastique Art finished parts for 11 CAE Air1 prototypes. Design adjustments were made through May. By June 16, the CAE Air1 ventilator was in its first round of certifications. By August 14, the second round of certifications was completed. On August 17, 142 days since the start of the project, mass production was under way. Under normal conditions, the average time for a medical device from conception to production typically takes at least 18 months.
To achieve the goal of making 10,000 certified units in three months, production had to be scaled to maximum capacity. Plastique Art invested in a new thermoformer and expanded their shifts to include weekends for two months. This enabled them to meet the goal of parts for 1,100 units per week.
Using SEKISUI KYDEX’s Quick Response Manufacturing Model, 50,000 pounds of KYDEX Thermoplastics were delivered every week to Plastique Art. The standard lead time of four weeks for material was expedited to one week. The volume of KYDEX Thermoplastics was so high, Plastique Art had to secure additional warehouse space to house the pallets of material.
“It was a privilege to work with Plastique Art and CAE on this important application, and our team was excited to provide whatever support was needed. CAE and Plastique Art are real-life heroes for making this happen, from concept to fulfillment, in a ridiculously fast time, which ultimately saved people’s lives,” said Mark Denning, medical market business manager, SEKISUI KYDEX. Thanks to a dedicated supply chain working together to make this project a success, the last of the CAE Air1 ventilators were completed in December 2020. www.kydex.com.